Switzerland – EPFL researchers have made a significant discovery that will aid in the design of renewable energy storage systems.
Scientists studied the behavior of catalysts at the particle level during water electrolysis. Catalysts are essential in this reaction, which splits water into hydrogen and oxygen.
If renewable energies are to one day replace fossil fuels, engineers must devise a reliable and large-scale storage system. Numerous researchers are currently investigating the possibility of storing energy in gaseous form inside electrolytic cells.
Electricity is used to initiate an electrolysis reaction that splits water molecules into hydrogen and oxygen. After that, the electricity can be recovered by reversing the reaction and recombining the hydrogen and oxygen into water.
Understanding catalysis
Catalysts are used to accelerate electrocatalytic reactions without being consumed. Metal oxides are used as catalysts in water electrolysis, with some performing better than others – though the exact reason is unknown.
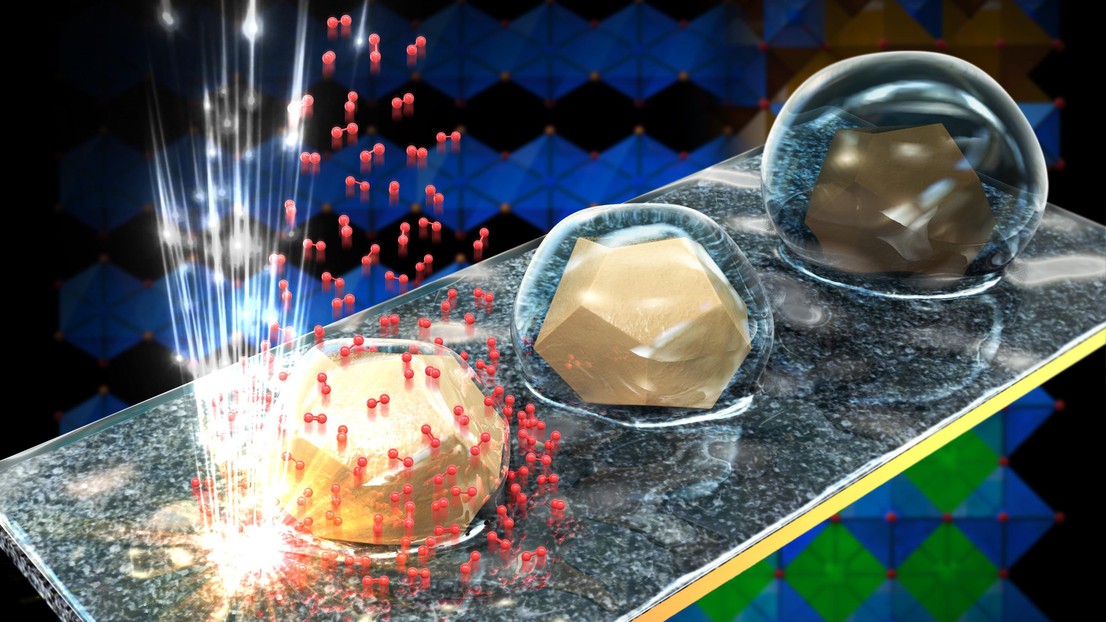
Tileli and Tzu-Hsien Shen, a PhD student in her lab, used an electron microscope to observe water electrolysis reactions and examine how the catalyst behaves throughout the process. Tileli and Shen photographed BSCF particles in real time at each stage of the electrolysis cycle. They observed the appearance of molecular oxygen, indicating that the reaction was taking place, and confirmed that the process was reversible.
Hydrophobic-to-hydrophilic transition
Furthermore, the researchers discovered that the surface atoms of the particles redistribute during the reaction, changing the surface properties. As a result, at different stages of the electrolysis cycle, the particles interact with their surroundings differently. During some steps, the surface is hydrophobic, while others are hydrophilic. The ability of a material to transition between hydrophobic and hydrophilic states is extremely valuable to engineers.