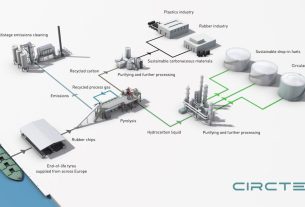
United States – A Rice University lab has discovered a chemical technique for converting waste plastic into a useful CO2 sorbent for industry.
When plastic waste was heated in the presence of potassium acetate, nanometer-scale pores formed, trapping carbon dioxide molecules. CO2 can be removed from flue gas streams using these particles.
Chemical recycling, a current method of pyrolyzing plastic, produces oils, gases, and waxes, but the carbon byproduct is nearly useless. When plastic is pyrolyzed in the presence of potassium acetate, porous particles are formed that can hold up to 18% of their own weight in CO2 at room temperature.
Furthermore, while traditional chemical recycling does not work for polymer wastes with low fixed carbon content in order to generate CO2 sorbent, such as polypropylene and high- and low-density polyethylene, which are the main constituents in municipal waste, when treated with potassium acetate, those plastics work especially well for capturing CO2.
The lab estimates that capturing carbon dioxide from a point source such as post-combustion flue gas would cost $21 per ton, far less than the energy-intensive, amine-based process that is currently used to extract carbon dioxide from natural gas feeds, which costs $80-$160 per ton.
Longer lasting
The sorbent, like amine-based materials, can be reused. When heated to about 75 degrees Celsius, trapped carbon dioxide is released from the pores, regenerating about 90% of the material’s binding sites.
Polyvinyl chloride vessels are sufficient to replace the expensive metal vessels that are normally required because they cycle at 75 degrees Celsius. The sorbent is expected to last longer than liquid amines, reducing downtime due to corrosion and sludge formation, according to the researchers.
To make the material, waste plastic is ground into a powder, mixed with potassium acetate, and heated for 45 minutes at 600 degrees Celsius (1,112 degrees Fahrenheit) to improve the pores, which are mostly 0.7 nanometers wide. Pores widened as the temperature rose. According to the researchers, the process also produces a wax byproduct that can be recycled into detergents or lubricants.