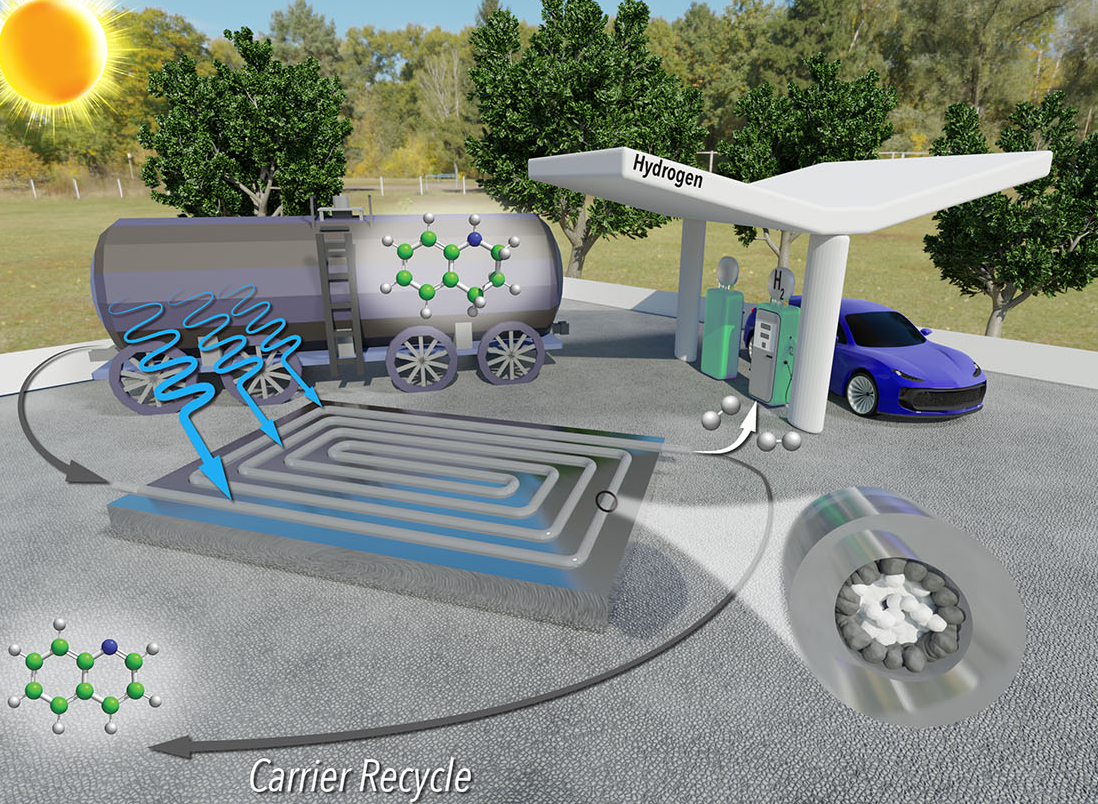
United States – North Carolina State University researchers have developed a new method for extracting hydrogen gas from liquid carriers that is faster, cheaper, and more energy efficient than previous methods.
CO2 emissions are not produced by hydrogen fuel. And, to take advantage of existing infrastructure, hydrogen refueling stations could be located at existing gas stations. However, because transporting hydrogen gas is hazardous, hydrogen must be transported in liquid form. Extraction of hydrogen from the liquid carrier at destination sites, such as fueling stations, is energy intensive and expensive, which is a major roadblock for this strategy.
The fact that the new technique is a continuous-flow reactor is one of the keys to its success. The reactor has the appearance of a thin, clear tube filled with sand. The “sand” is made up of micron-sized titanium oxide grains, many of which are rhodium-coated. One end of the tube is pumped with hydrogen-carrying liquid. The rhodium-coated particles line the tube’s outer edge, where they can be exposed to sunlight. These particles are photoreactive catalysts that react with the liquid carrier to release hydrogen molecules as a gas when exposed to sunlight.
99 percent yield
The researchers meticulously designed the system so that only the outer grains of titanium oxide are coated with rhodium, ensuring that no more rhodium is used than is required. In three hours, the researchers were able to achieve a 99 percent yield in their prototype reactor, which means that 99 percent of the hydrogen molecules were released from the liquid carrier.
The flow system can run for up to 72 hours before its efficiency starts to deteriorate. The catalyst can now be “regenerated” without having to remove it from the reactor; it’s a quick cleaning procedure that takes about six hours. The system can then be restarted and run for another 72 hours at full efficiency. The technology has been granted a provisional patent by NC State.